Jill Stein, a medical doctor who is running for President on the Green Party ticket, has claimed that the pharmaceutical industry has a corrupting influence on the Food and Drug Administration (FDA). She has also claimed that she was part of a public health movement that led to the removal of mercury from childhood vaccines. In reality, we have no evidence that the mercury in childhood vaccines was causing any harm. Nor was any grassroots organization, other than the American Academy of Pediatrics, involved in the decision to stop using a mercury compound called thimerosal as a preservative in vaccines. (Stein is an internist, not a pediatrician.) The decision to make childhood vaccinations mercury-free was made by the FDA and the Centers for Disease Control and Prevention (CDC). Prominent antivaccination activists started speaking out about mercury in vaccines only after the vaccines became mercury-free.
It is disturbing that Republican and Green Party Presidential hopefuls, including some medical doctors, have been using the talking points of the antivaccination movement. What’s worse is that any medical doctor, and especially any medical doctor who wants to be chief executive of the federal government, does not seem to know how the federal government works to protect public health.
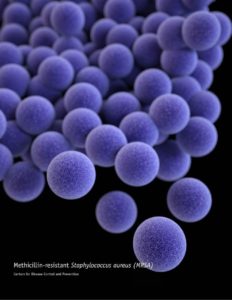
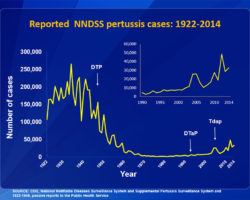
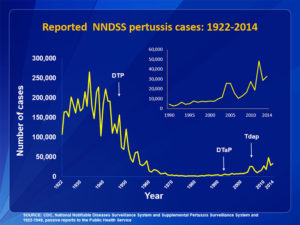
Many laymen were horrified to hear that a mercury compound was ever being used as an ingredient in childhood vaccines. Yet that mercury compound is a powerful preservative that was being used to solve a serious safety problem. This problem became obvious in 1928, in a disaster called the Bundaberg Tragedy. A bottle of diphtheria vaccine in a doctor’s office in Bundaberg, Queensland, Australia, became contaminated with a bacterium called golden staph (Staphylococcus aureus). The bacterium was probably carried into the bottle by the needle that was used to draw out one of the first doses from the bottle. Then, the bacteria grew inside the bottle as it sat on a shelf between doses. Twelve of the children who received vaccine from this contaminated bottle died. Five others became seriously ill but recovered. To prevent a similar tragedy from happening in the United States, the US Code of Federal Regulations (21CFR610.15) requires vaccine makers to put a preservative in multiple-dose containers of practically all vaccines. Single-dose containers can be preservative-free but are more expensive.
The regulation does not specify which preservatives must be used. However, it does say that the preservative must be “sufficiently nontoxic so that the amount present in the recommended dose of the product will not be toxic to the recipient.” Also, the preservative must not interfere with the potency of the vaccine. Thimerosal has been used since the 1930s as a preservative in vaccines because it was the most effective option, it did not interfere with the potency of the vaccine, and it was well tolerated. Thimerosal has also been used as a preservative in contact lens solutions. Even today, despite an extensive research effort, we have no evidence that the use of thimerosal in vaccines has caused any health problems.
The person who raised the question of the mercury content of medicines (not specifically vaccines) was Frank Pallone, a Democratic Congressman from New Jersey. In 1997, he introduced an amendment to the FDA’s reauthorization bill. This amendment gave FDA two years to compile a list of all medicinal products that contain mercury compounds as ingredients. The FDA had to analyze what kind of mercury compound was in each product, and how much of each mercury compound the product contains. In response to this Congressional mandate, the FDA revisited the question of how much exposure children were getting to thimerosal through their vaccinations.
Because of the introduction of some new vaccines, the amount of thimerosal that children were receiving had gone up. In 1999, scientists at the FDA calculated that the recommended vaccines would deliver a total of 187.5 micrograms of mercury. (A microgram is a millionth of a gram.) However, there was no reliable way to judge whether this amount of mercury exposure is a problem. In the human body, thimerosal is broken down into ethylmercury, but the federal guidelines on mercury toxicity were based on methylmercury. To be on the safe side, the scientists assumed that ethylmercury would be just as dangerous as methylmercury. (We now know that it is not, because it is quickly eliminated through the kidneys.) So they suggested that steps be taken to reduce thimerosal exposure. Back in 1999, we had no evidence that the thimerosal in vaccines was causing problems. By now, we have evidence that it was not causing any of the health problems that were investigated. However, it did become a serious public relations problem.
The FDA and the CDC take vaccine safety seriously. As a result, the recommended vaccines are amazingly safe. Yet there is one thing that we can do to improve safety still further, while restoring public trust in the public health authorities. We must focus on driving diseases like polio, measles, and rubella into extinction through vaccination. Once a disease is extinct, everyone is protected against it, forever. As a result, children do not need to be exposed to even the minimal risks, and the discomfort, of the vaccination. Instantly, the sales of the vaccine drop to zero. By working to eradicate a vaccine-preventable disease, we public health activists make it crystal clear that our goal is public health, not private profit.




 A measles virus infection starts off as a respiratory infection. Thus, it starts off looking and feeling like a common cold. But then it takes a dangerous turn: it infects the immune system. Measles can cause long-lasting damage to the immune system. Recent studies have shown that a case of measles can increase a child’s risk of death from other infections for
A measles virus infection starts off as a respiratory infection. Thus, it starts off looking and feeling like a common cold. But then it takes a dangerous turn: it infects the immune system. Measles can cause long-lasting damage to the immune system. Recent studies have shown that a case of measles can increase a child’s risk of death from other infections for